Now Reading: Scientists Achieve Breakthrough in Stabilizing 48-Atom Carbon Ring
-
01
Scientists Achieve Breakthrough in Stabilizing 48-Atom Carbon Ring
Scientists Achieve Breakthrough in Stabilizing 48-Atom Carbon Ring
Fast Summary
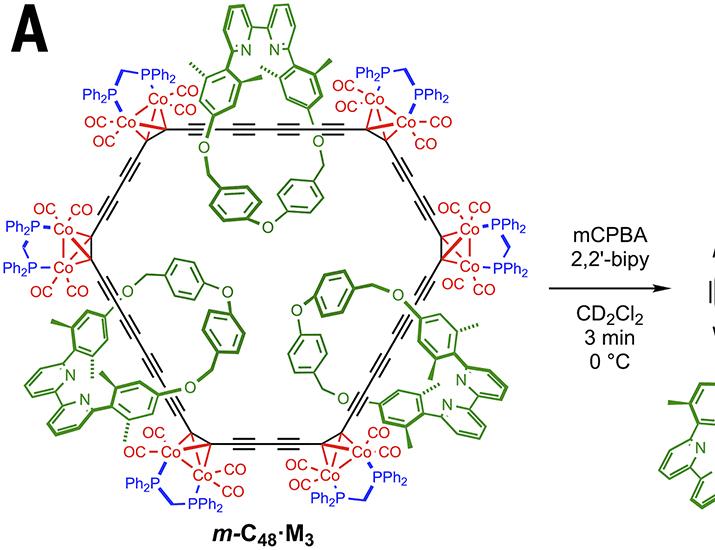
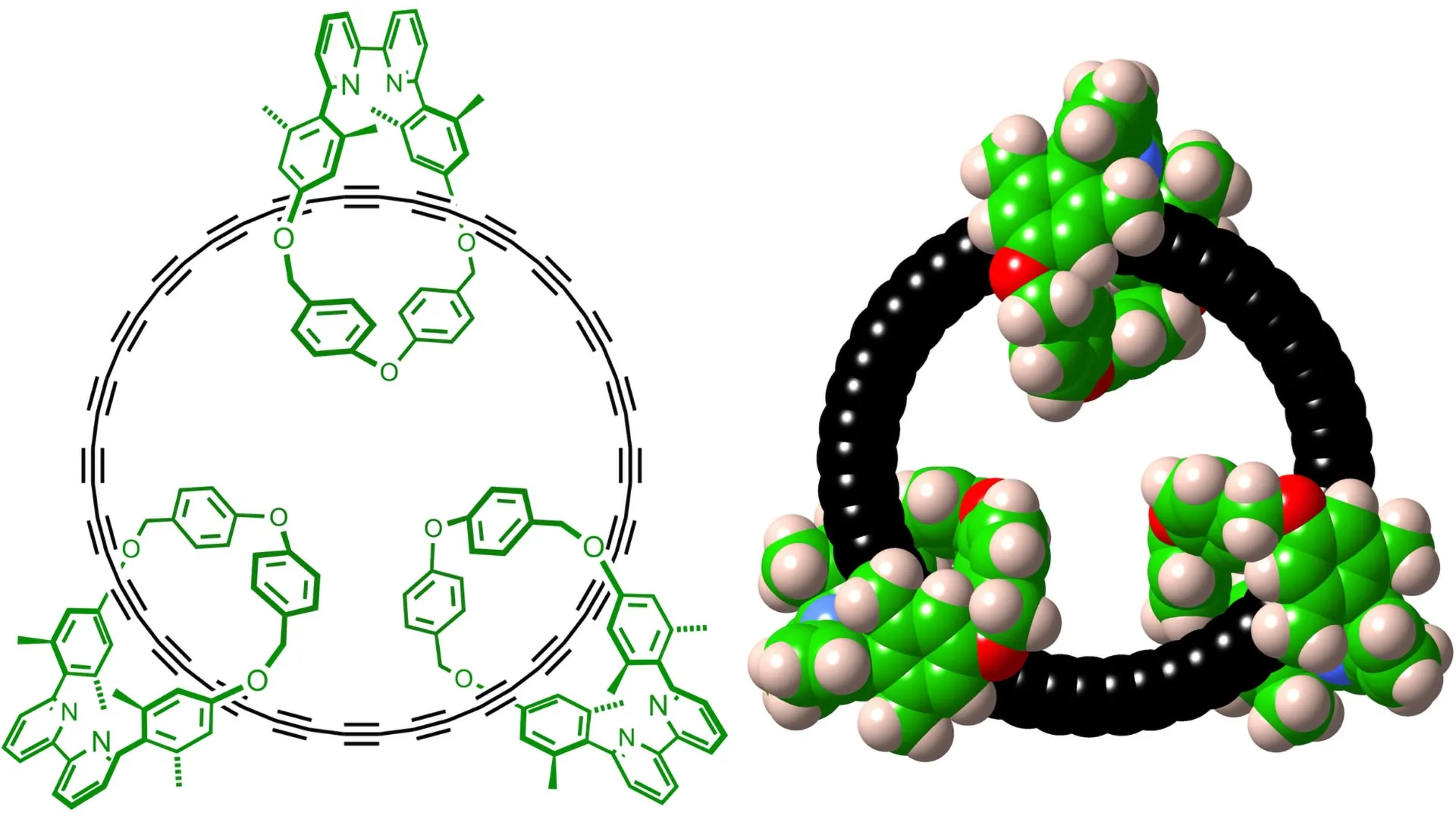
- Chemists at Oxford University have successfully synthesised a stable cyclocarbon that can be studied in liquid solution at room temperature (20°C).
- The molecule,named cyclo[48]carbon,was stabilised by threading it through three macrocycles,creating a [4]catenane structure.
- Previous molecular carbon rings were studied only in the gas phase or under extremely low temperatures (4-10 K).
- Cyclo[48]carbon maintains stability with a half-life of 92 hours and was characterised using mass spectrometry, NMR, UV-visible spectroscopy, and Raman spectroscopy.
- Strong evidence for the novel structure came from findings such as identical environments for all 48 sp¹ carbon atoms observed through NMR resonance.
- Researchers emphasise this breakthrough enables easier study of cyclocarbons’ properties and reactivity under laboratory conditions.
- The study is published in Science journal and involved collaborators from multiple UK institutions including the Universities of Manchester and Bristol.
Indian Opinion Analysis
The synthesis of cyclo[48]carbon marks an extraordinary advancement in materials chemistry with implications for fundamental research on carbon-based molecular structures. For India’s scientific community, such breakthroughs underscore the importance of adopting robust interdisciplinary collaboration models like those seen in oxford’s effort-essential for solving complex challenges over extended periods.
India’s emphasis on clean energy innovations linked to carbon nanostructures coudl benefit from deeper engagement or adaptation based on discoveries like this one. Universities across India can consider developing state-of-the-art facilities akin to Oxford’s NMR capabilities to enable cutting-edge research within domestic labs-perhaps positioning Indian scientists as contributors to foundational achievements globally.
Link: Read More