Now Reading: Indian Scientists Capture Quantum Motion of Molecule in Breakthrough Imaging
1
-
01
Indian Scientists Capture Quantum Motion of Molecule in Breakthrough Imaging
Indian Scientists Capture Quantum Motion of Molecule in Breakthrough Imaging
Quick Summary
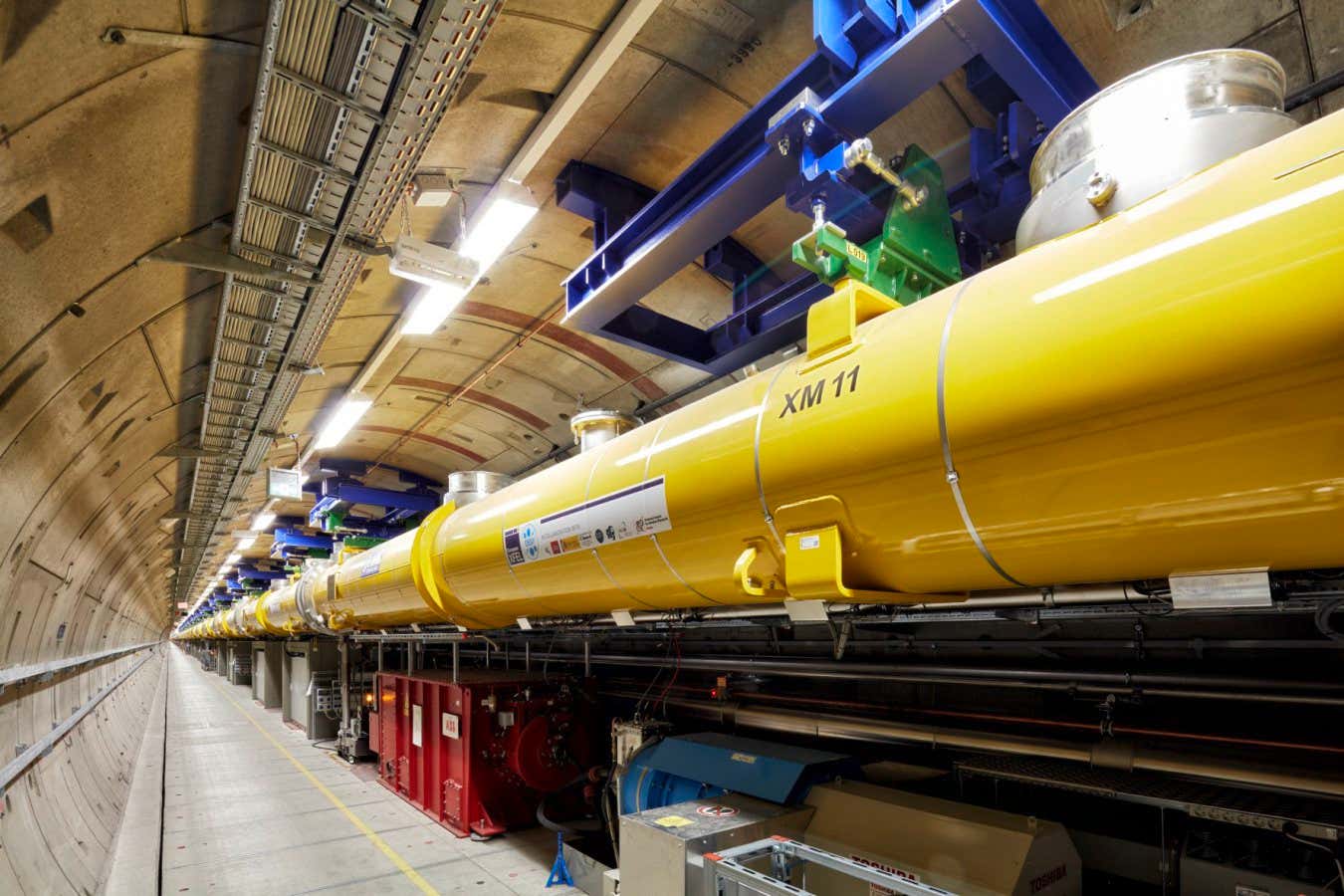
- Researchers at the European XFEL laser facility in Germany, led by Till Jahnke, used a powerful X-ray laser to observe atomic motions in an 11-atom molecule composed of carbon, hydrogen, nitrogen, and iodine.
- The atoms exhibited slight synchronized movements known as “Heisenberg jiggle,” stemming from quantum mechanics principles such as Heisenberg’s uncertainty principle.
- The X-ray pulses were incredibly luminous-millions of times brighter than medical X-rays-and extremely brief at quadrillionths of a second.
- This technique allowed scientists to reconstruct the atom-level quantum fluctuations at their lowest energy state with unprecedented precision.
- They observed these movements follow a synchronized choreography tied to molecular structure but were surprised by how accurately they could measure it.
- Future goals include applying these methods to larger systems and studying quantum fluctuation impacts on chemical reactions and electron behavior.
Image Caption: The accelerator tunnel of European XFEL where measurements took place (Credit: XFEL/Heiner Mueller-Elsner).
campaign=RSS%7CNSNS&utmsource=NSNS&utmmedium=RSS&utmcontent=home”>Read More
Stay Informed With the Latest & Most Important News
Previous Post
Next Post
Loading Next Post...




























